mRNA Vaccines: Key questions and answers
In recent years, messenger RNA (mRNA) vaccines have become a central topic in biotechnology and public health. This type of vaccine, brought into the spotlight by its role in the COVID-19 pandemic, represents an innovative approach to disease prevention and treatment. Below, we present an article structured around frequently asked questions about mRNA vaccines, with answers based on up-to-date scientific information. Each section addresses a common concern, from how they work to their safety, and explores the future of this technology.
What vaccines use mRNA?
Currently, the only mRNA vaccines approved for human use are those developed against COVID-19. Specifically, the Comirnaty vaccine by Pfizer-BioNTech and Spikevax by Moderna use mRNA technology to induce immunity against SARS-CoV-2. These vaccines have been administered to hundreds of millions of people worldwide with high effectiveness in preventing severe COVID-19.
Other companies have also worked on mRNA vaccines. For example, the German company CureVac was a pioneer in researching mRNA vaccines for rabies, influenza, and other diseases, although its first COVID-19 candidate did not reach the desired efficacy. In China, companies like Walvax have developed mRNA vaccine candidates against COVID-19, and global pharmaceutical companies (such as Sanofi or GSK) have invested in this technological platform. This reflects a broad global interest in mRNA vaccines as a new tool to fight infectious diseases.

What does mRNA do in the body?
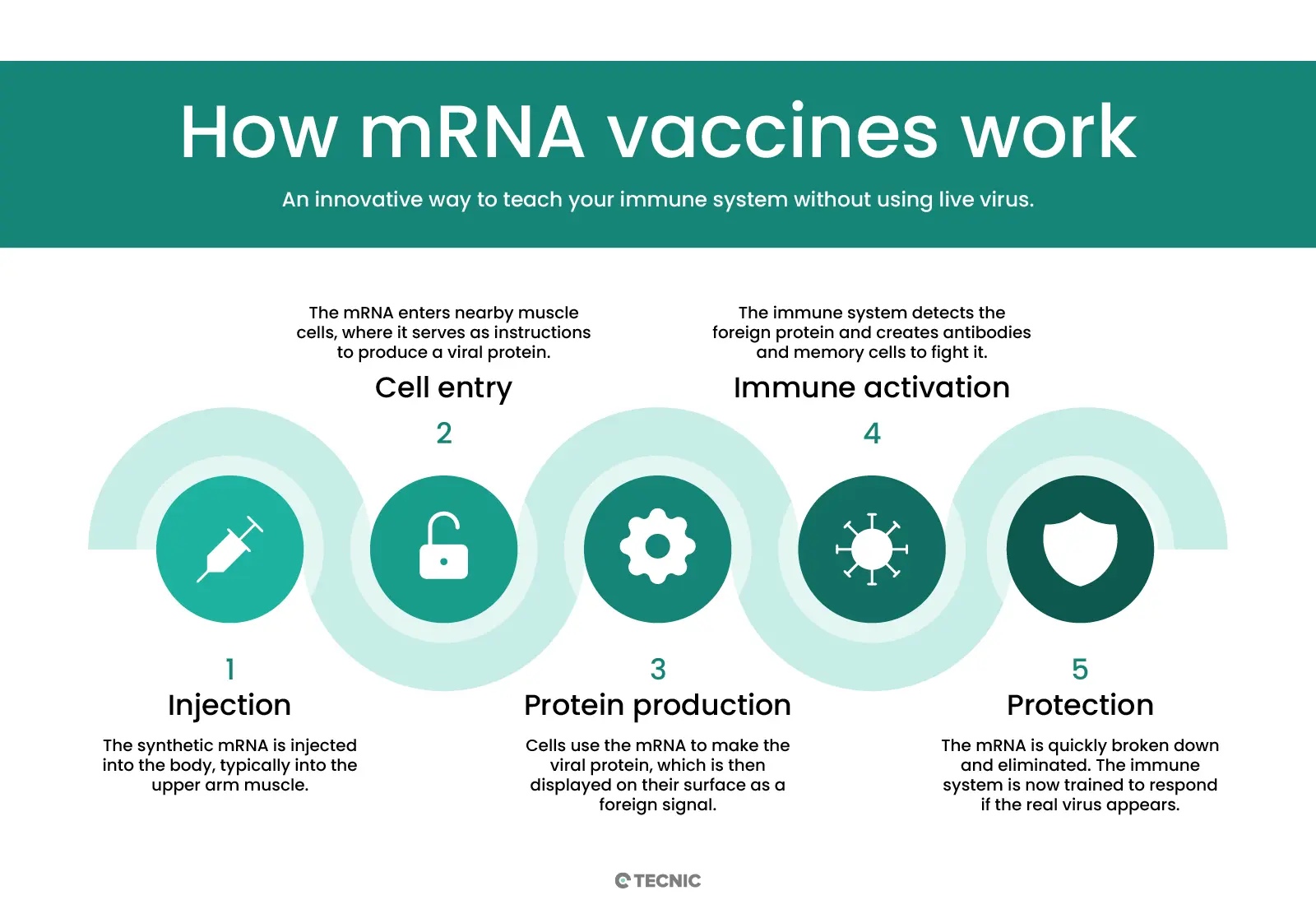
mRNA vaccines work differently from traditional vaccines. Instead of introducing a weakened or inactivated virus, or a viral protein, these vaccines deliver a synthetic messenger RNA fragment that contains the instructions to produce a specific protein from the pathogen. Once the mRNA enters our cells (especially muscle cells near the injection site), it acts as a template for the cell to produce the target viral protein, for example the spike protein of the coronavirus.
By producing this foreign protein, the immune system recognizes it as a threat and mounts a defensive response: it generates specific antibodies and immune cells trained to attack the real pathogen. It's important to emphasize that the vaccine mRNA does not enter the cell nucleus or alter human DNA. Furthermore, after fulfilling its role, the mRNA is quickly broken down by the cell and eliminated from the body. Thus, the vaccine enables our body to practice and prepare its defenses without exposing us to the whole virus or causing disease. In essence, the mRNA in the body acts as a temporary messenger that teaches our immune system how to protect itself, and then disappears.
Is the mRNA vaccine safe?
Yes. mRNA vaccines have shown a very favorable safety profile. Like other vaccines, they underwent rigorous clinical trials and received approval from regulatory agencies such as the FDA (in the U.S.) and EMA (in Europe) after confirming their safety and efficacy. Millions of people have been vaccinated with mRNA formulas in recent years, and ongoing monitoring has found no widespread or long-term adverse effects linked to these vaccines. In fact, experts state it is far more likely to suffer serious consequences from contracting a preventable disease than from vaccination itself.
There are clear biological reasons for their safety. First, mRNA vaccines do not contain live virus, so they cannot cause the disease they are meant to prevent. Additionally, the mRNA does not interfere with our genome: it does not enter the cell nucleus or modify DNA, and it remains in the body only long enough to generate immunity. The most common side effects are similar to those of other vaccines (see the side effects section) and tend to be mild and short-lived. Severe adverse effects, such as strong allergic reactions or heart inflammation, are extremely rare and have occurred mainly in specific populations, typically with a benign clinical course. In short, based on the available evidence, mRNA vaccines are considered safe and a crucial tool for public health.
How long does it stay in the body?
A common concern is how long the vaccine’s mRNA molecule remains in the body. The answer is very little time. mRNA is, by nature, an unstable and short-lived molecule; outside cells, it quickly degrades. After vaccination, the mRNA stays in the body only long enough for cells to produce the target protein and activate the immune system, a process that takes a few days at most.
Our bodies have enzymes and mechanisms designed to break down messenger RNA once it has been used. In fact, inside cells, mRNA is constantly recycled: after producing the instructed proteins, it is destroyed and eliminated. Studies and follow-up data have confirmed that vaccine mRNA does not persist long-term in any tissue. What it leaves behind is the trained immune response (antibodies and memory cells), which can last for months or years, protecting us from illness. Therefore, the mRNA molecule fulfills its mission and then quickly disappears, leaving no trace in the body.
What diseases can mRNA treat?
The mRNA vaccine platform opens the door to addressing multiple diseases, both infectious and non-infectious. In the realm of infectious diseases, mRNA can be adapted to encode proteins from various viruses and pathogens, theoretically allowing vaccines for many illnesses. It has already proven effective against COVID-19, and experimental mRNA vaccines have been studied for influenza, rabies, Zika virus, respiratory syncytial virus (RSV), among others. While many of these vaccines are still in the research phase, early results suggest that mRNA is a versatile platform for preventing diseases caused by very different viruses.
Beyond infections, mRNA vaccines can also be used in therapeutic applications. A prominent example is their potential in cancer treatment. For years, scientists have explored personalized mRNA vaccines that help the immune system recognize and attack specific tumors. Clinical trials are underway for mRNA vaccines targeting various cancers (like melanoma, pancreatic cancer, or colorectal cancer), sometimes in combination with immunotherapies to enhance the body’s response. Although no mRNA cancer vaccines have been approved yet, early results are promising and suggest this technique could lead to new types of therapeutic vaccines in the future.
In short, mRNA has the potential to tackle a wide range of diseases. Any condition where a key protein target can be identified (whether viral, bacterial, or an abnormal protein in cancer cells) can potentially be addressed with an mRNA vaccine that instructs the body to fight it. This includes emerging infections as well as complex conditions like cancer, marking an exciting frontier in preventive and personalized medicine.
What was the first mRNA vaccine?
Although mRNA vaccine research has been ongoing for decades, the first mRNA vaccine approved for general population use was the COVID-19 vaccine developed in 2020. Specifically, the Pfizer-BioNTech vaccine (Comirnaty) received emergency use authorizations in late 2020 in several countries (such as the United Kingdom and the United States), becoming the first mRNA vaccine used on a large scale in humans. It was closely followed by Moderna’s vaccine (Spikevax), approved in early 2021. Both drugs marked a historic milestone, as they were the first real-world proof of this technology’s effectiveness in disease prevention.
Interestingly, before the COVID-19 pandemic, clinical trials for mRNA vaccines had already been conducted, though none resulted in licensed vaccines. For instance, between 2013 and 2017, experimental mRNA vaccines for rabies were tested in humans, and animal trials were conducted for diseases like Ebola. However, the global health emergency in 2020 dramatically accelerated the development and approval of an mRNA vaccine. As the European Medicines Agency stated, “the first mRNA vaccine approved in Europe was in 2020 against COVID-19.” This event marked the beginning of a new era in vaccinology.

What are the side effects?
Like any vaccine or medication, mRNA vaccines can cause side effects, though in the vast majority of cases, they are mild and short-lived. The most common adverse effects linked to mRNA vaccines (mainly identified with the COVID-19 vaccines from Pfizer-BioNTech and Moderna) include: pain or tenderness at the injection site, redness or swelling in the arm, fatigue, headache, low-grade fever, chills, and muscle or joint pain. These symptoms are usually signs of the immune system being activated and typically resolve on their own in a day or two. Many people experience only mild local discomfort.
Serious side effects are rare. Severe allergic reactions (anaphylaxis) are possible but extremely rare, which is why a 15–30-minute observation period after the shot is recommended as a precaution. Another rare effect associated with mRNA COVID-19 vaccines is myocarditis or pericarditis, which is inflammation of the heart or surrounding tissue. This effect has been observed mainly in young males after the second dose and occurs at a very low frequency (around a few dozen cases per million vaccinated). Moreover, reported cases have typically been mild, responded well to treatment, and resulted in full recovery for most patients. Regulatory bodies such as the Spanish Agency for Medicines and Health Products (AEMPS) have concluded that myocarditis related to the vaccine is very rare, and that the benefits of vaccination far outweigh this infrequent risk.
In summary, the side effects of mRNA vaccines are similar to those of other modern vaccines. Most people will experience mild, temporary symptoms or none at all, and only a very small proportion will experience serious effects. Global monitoring over the past years strongly supports the safety of these vaccines, and any rare risks are carefully tracked by health authorities.
What brands manufacture mRNA vaccines?
Several leading biotechnology and pharmaceutical companies are behind the development and manufacturing of mRNA vaccines. The most well-known brands are, without a doubt, Pfizer-BioNTech and Moderna. Pfizer (a U.S.-based multinational) partnered with the German biopharma company BioNTech to create the Comirnaty vaccine against COVID-19, while Moderna (a U.S. company) developed the Spikevax vaccine independently. Both organizations leveraged years of prior mRNA research and, in record time, produced the first mRNA vaccines approved during the pandemic.
Another pioneering company is CureVac, a German start-up that has focused on mRNA technology for over 20 years. CureVac conducted early clinical trials for mRNA vaccines against rabies and other viruses before 2020. Although its first COVID-19 vaccine did not achieve optimal efficacy results, it continues developing a second-generation mRNA vaccine in partnership with other firms.
In addition to these, pharmaceutical giants such as Sanofi (in alliance with Translate Bio) and GlaxoSmithKline (GSK) have invested in mRNA vaccine projects for influenza and other diseases. In Asia, companies like Walvax and Abogen in China have developed their own mRNA vaccines against the coronavirus. Governments and international coalitions, supported by the WHO, are also promoting technology transfer centers so that more countries can produce mRNA vaccines.
In short, while Pfizer-BioNTech and Moderna are the flagship brands associated with mRNA vaccines, there is an entire ecosystem of innovative companies (CureVac, BioNTech, Moderna, among others) and traditional pharmaceutical giants entering this space. This global collaboration is expanding the production capacity and reach of mRNA vaccines worldwide.
How were these vaccines developed?
mRNA vaccines may seem like a sudden breakthrough, but they are actually the result of decades of scientific research. Messenger RNA was discovered in 1961, laying the theoretical foundations for its use. However, for a long time, it was considered too unstable for medical applications: it's a single-stranded molecule that degrades quickly outside controlled environments. To use mRNA as a vaccine, scientists had to overcome several challenges:
Stability of mRNA: In the 1970s, a key breakthrough was made by encapsulating mRNA in a lipid layer, which protects it from degradation. This fatty coating forms a nanoparticle that allows mRNA to reach the inside of cells intact.
These lipid nanoparticles protect the mRNA and facilitate its delivery into cells. This innovation was essential to overcome the natural fragility of mRNA, since without protection, the body would destroy it before it could function. Encapsulating it in lipid nanoparticles enables mRNA to enter cells and direct protein production before degrading.
Synthetic mRNA production: In the 1980s and 1990s, significant advances were made. Scientists learned to produce mRNA molecules in the lab and modify them slightly to improve performance. In 2005, researchers like Katalin Karikó introduced chemical modifications (such as pseudouridine) to synthetic mRNA to make it less visible to the innate immune system, avoiding adverse reactions and boosting protein expression. This solidified the viability of modern mRNA vaccines.
Pre-pandemic trials: Between 2009 and 2019, early-stage clinical trials were conducted for mRNA vaccines against diseases like rabies, HIV, influenza, and various cancers. These small-scale studies showed that mRNA vaccines could elicit immune responses in humans. Small biotech firms like BioNTech and CureVac were already testing mRNA cancer vaccines before 2020, building knowledge on formulation and dosing.
Pandemic as a catalyst: The arrival of the COVID-19 pandemic in late 2019 and early 2020 was the major catalyst. The scientific community, which had been fine-tuning mRNA technology for years, could rapidly design vaccines against the new coronavirus. Within days of obtaining the SARS-CoV-2 genetic sequence, researchers created vaccine candidates almost immediately. No vaccine had ever been developed this quickly: the design, production, and early safety/efficacy testing was completed in under a year—when it normally takes a decade or more. This was possible due to previous research and unprecedented collaboration between research centers, companies, and health authorities.
Thus, mRNA vaccines were developed by combining scientific persistence and technological innovation over more than 30 years. Far from being a rushed invention, they are the result of many iterations and learnings: from how to protect the molecule, to how to manufacture it at scale. The pandemic simply offered the opportunity to prove their potential on a global stage, validating decades of effort in labs around the world.

Katalin Karikó is a Hungarian biochemist renowned for her research on messenger RNA (mRNA).
What diseases are being studied for mRNA vaccine use?
Following the success against COVID-19, attention has turned to harnessing mRNA technology for other diseases. Currently, mRNA vaccines are being researched for several high-impact infectious diseases as well as complex health problems. The conditions under investigation include:
Emerging infectious diseases: Clinical trials are underway for mRNA vaccines targeting viruses such as seasonal influenza, respiratory syncytial virus (RSV), and Zika virus, among others. These pathogens represent persistent or re-emerging threats, and mRNA vaccines could provide faster responses to mutations or new outbreaks.
HIV/AIDS: The human immunodeficiency virus has eluded an effective vaccine for decades. mRNA’s flexibility offers a new path to attempt vaccines against HIV by designing sequences that train the immune system to neutralize this highly variable virus. Several institutions, including the HIV Vaccine Initiative, are working on mRNA-based HIV vaccine prototypes.
Malaria and Tuberculosis: These are two of the deadliest infectious diseases globally. Projects are already underway to develop mRNA vaccines against malaria and tuberculosis, aiming to improve or complement existing traditional vaccines. For instance, BioNTech launched a phase I trial for a malaria mRNA vaccine in 2022, and a TB mRNA vaccine is being explored focusing on key proteins from Mycobacterium tuberculosis.
Cancer: Beyond the therapeutic vaccines already mentioned, researchers are exploring mRNA use to prevent cancers caused by viruses (such as a potential vaccine to prevent cancers linked to the Epstein-Barr virus, or enhancements to the HPV vaccine). Likewise, personalized mRNA cancer vaccines are being tested to teach cancer patients’ immune systems to attack specific tumor mutations.
Other applications: Scientists are exploring whether mRNA techniques could be used to induce tolerance in autoimmune diseases or regenerate tissues via growth factors. There is also interest in non-vaccine mRNA therapies, such as treating genetic diseases by supplying mRNA for a functional protein the patient cannot produce. These uses are still in early stages but reflect the wide range of possibilities.
In conclusion, mRNA vaccine research spans from classic infectious diseases (flu, HIV, malaria, etc.) to frontiers like cancer and rare diseases. The speed at which an mRNA can be designed for a new target has the scientific community optimistic about its potential for future vaccines. Many trials are underway worldwide, and it is likely that in the coming years we will see the first non-COVID-19 mRNA vaccines become a reality.
How does it differ from other vaccines?
mRNA vaccines differ fundamentally from other traditional vaccine types (such as inactivated viruses, attenuated viruses, or protein subunit vaccines):
Mechanism of action: The key difference is what is introduced into the body. Classical vaccines often deliver the antigen directly (for example, a viral protein) or even the whole weakened/inactivated pathogen. In contrast, mRNA vaccines deliver only the genetic blueprint (mRNA) so our own cells can produce the harmless antigen. That is, they turn the body into the "factory" for the immunogenic protein. This eliminates any risk of infection from the vaccine, as no live virus is injected.
Speed and adaptability: mRNA vaccines are quick to design and manufacture compared to others. Once the genetic sequence of a pathogen is known, the corresponding mRNA can be synthesized in weeks. There’s no need to grow viruses in eggs or cells for months, as with some traditional vaccines. This "genetic code" platform enabled vaccine development at record speed during the pandemic (under a year, when >5–10 years is typical). Additionally, if a virus mutates, updating an mRNA vaccine is as simple as modifying the mRNA sequence—a much faster process than reformulating conventional vaccines.
Composition and manufacturing: Other vaccines often contain chemical adjuvants to boost the immune response; mRNA vaccines typically do not need them, as the delivery of mRNA (and the resulting protein) naturally stimulates the immune system. mRNA vaccines are formulated with lipid nanoparticles (microscopic fat droplets) that protect the mRNA and help it enter cells—something we don’t see in traditional vaccines. From a manufacturing perspective, producing mRNA is a synthetic biotech process (in vitro transcription), different from virus culture or protein purification, and can be rapidly scaled once optimized.
Storage: A practical difference is that early mRNA vaccines required ultra-cold storage (e.g., -70°C for Pfizer-BioNTech) due to mRNA fragility. In contrast, many conventional vaccines are kept refrigerated (2–8°C). However, new formulations are being developed to improve mRNA vaccine stability. mRNA vaccines also typically come in frozen multi-dose vials, whereas others may be single-dose and ready to use.
In summary, mRNA vaccines differ through their genetic-based approach: instead of supplying the antigen or the germ, they deliver the instructions to make it. This provides advantages in development speed and strong immune response but also challenges such as initial cold-chain requirements. Despite the differences, the ultimate goal remains the same as with any vaccine: to safely and effectively prepare the immune system to defend against a pathogen.

Conclusion
mRNA vaccines have revolutionized immunology by demonstrating a new way to protect health. Their rapid development and adaptability make them a valuable tool against pandemics and other difficult diseases. Although challenges remain (like improving their stability and expanding their use to more conditions), success against COVID-19 has validated decades of research and opened a promising path for future vaccines and genetic therapies.
In this progress, the role of the biotech industry and its technology providers is essential. Companies like TECNIC have driven biotechnology forward by providing essential tools and equipment to bring these vaccines from the lab to large-scale production. For example, TECNIC’s bioreactors and tangential flow filtration (TFF) systems enable optimal cell culture and efficient mRNA production, ensuring a smooth transition from research to industrial-scale manufacturing. These technological innovations ensure that mRNA vaccines can be manufactured safely, quickly, and in sufficient quantities to meet global needs. As TECNIC highlights, its bioreactors can be tailored to the specific demands of mRNA vaccines, reflecting a commitment to excellence and innovation in public health.
In short, the convergence of scientific breakthroughs and support from specialized companies has made mRNA vaccines a reality today. As we continue exploring new applications (from cancer to malaria) and improving these vaccines, collaboration between science and industry will be key. Thanks to this synergy, we are moving toward a future where disease prevention and treatment will be more agile, accessible, and effective than ever before.

Frequently Asked Questions about mRNA Vaccines
It is a vaccine that uses messenger RNA instructions to help your cells produce a viral protein and trigger an immune response against it.
Yes. They have been widely studied and administered to millions of people, showing a strong safety profile and mostly mild, short-term side effects.
Currently they prevent COVID-19, but vaccines are being developed for flu, rabies, HIV, RSV, malaria, tuberculosis, and even certain types of cancer.
No. mRNA does not enter the cell’s nucleus and does not interact with your DNA. It is broken down naturally by the body shortly after doing its job.
Just a few days. Once the viral protein is produced, the body quickly degrades and eliminates the mRNA through natural processes.
References
National Cancer Institute. (2022, February 11). Can mRNA Vaccines Help Treat Cancer? National Institutes of Health.
European Centre for Disease Prevention and Control. (n.d.). COVID-19 vaccination.
- UK Health Security Agency. (2024, March 11). What are mRNA vaccines and how do they work?
- Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261–279.
- World Health Organization. (n.d.). mRNA vaccines: Norms and standards.
- Zhang, C., Maruggi, G., Shan, H., & Li, J. (2019). Advances in mRNA vaccines for infectious diseases. Frontiers in Immunology, 10, 594.
How to cite this article
TECNIC (2025). What is an mRNA vaccine and how does it work?. Retrieved from https://www.tecnic.eu/what-is-an-mrna-vaccine-and-how-does-it-work/







